
Did you know that water and air are simply chemical compositions? Those probably aren’t the first two chemicals that you think of when someone mentions the word chemical.
Chemicals don’t have to be dangerous or harmful. Your entire body is composed of many chemicals, and the majority of the chemicals that you encounter on a daily basis are perfectly safe.
One of the least understood chemical properties within the natural hair community is potential hydrogen or pH.
Even though pH balancing is often mentioned when people are looking for new hair care products, it’s rarely well understood within the natural hair community. Let’s begin by talking about the abbreviation for Potential Hydrogen (pH).
Table of Contents
- 1 What Is the pH of Hair?
- 2 The pH Scale Is a Logarithmic Scale
- 3 Healthy pH Balance and Hair Health
- 4 How to Test pH of Hair Products
- 4.1 While Retesting Hair Products, Why Would I Receive Different pH Readings? Are the pH Strips Faulty?
- 4.2 How Accurate Are pH Strips?
- 4.3 Should I Purchase pH Strips That Provide Half Values? Are These Potential Hydrogen Strips Better?
- 4.4 Testing the pH of Hair Conditioners
- 4.5 Since Hair Isn't a Liquid, How Do You Test the pH of Your Hair?
- 4.6 Does pH Raise or Close Cuticles?
- 4.7 Can High pH Levels Prevent the Cuticle from Opening?
- 4.8 What Happens When pH Is Below or Above the 3 to 9 Range?
- 4.9 Are You Able to Test the pH of a Substance Such as Coconut Oil?
- 4.10 Does the pH of Hair Shift After a Person Uses Soap?
- 4.11 How Long Is the Recovery Time After Exposing Your Hair or Skin to Alkaline pH?
- 4.12 Do You Need to Worry About Irritation When Using Castile Soap or Baking Soda With an Alkaline pH?
- 4.13 Is Baby Shampoo pH Balanced?
- 4.14 Related Articles
What Is the pH of Hair?
The pH of the hair shaft is around 3.67, while the pH of the scalp is 5.5. The more acidic pH of the hair shaft helps to maintain the structural integrity of the hair, keeping the cuticle layer closed and protecting the hair from damage.
In contrast, the slightly acidic pH of the scalp is important as it helps to protect the skin and hair from harmful bacteria and fungi.
Natural pH Balance
Understanding the hair's pH can help you choose the right hair products and treatments, as using products with an incorrect pH level can lead to damaged hair, including cuticle damage, and other issues.
The term pH represents the number of hydrogen ions, where p represents the quantity and H stands for hydrogen ion. An ion is a molecule or an atom that carries an electrical charge. When a molecule or atom separates into positive or negative ions, the process is called ionization.
An ion that carries a negative electrical charge is called an anion. On the other hand, an ion that carries a positive electrical charge is called a cation. The pH scale is simply a measurement of these ions.

pH Balanced Products
Your hair products must contain water in order for them to have a pH.
Let’s use water (H20) as an example to more fully understand potential hydrogen (pH). In H20, some of the molecules will naturally ionize into hydrogen ions (H+ acidic) and hydroxide ions (OH- alkaline).
The more hydrogen ions that a hair product has, the more acidic the product will be. Conversely, the more hydroxide ions that a hair product has, the more alkaline the product will be.
Distilled water (pure water), which is neutral on the pH scale, has a pH of 7.
This means that distilled water has an equal balance of 50 percent hydrogen ions (acidic) and 50 percent hydroxide ions (alkaline).
As the percentage of acidity increases in a product, the percentage of alkalinity decreases. The reverse is also true. As the percentage of alkalinity increases in a product, the percentage of acidity decreases.
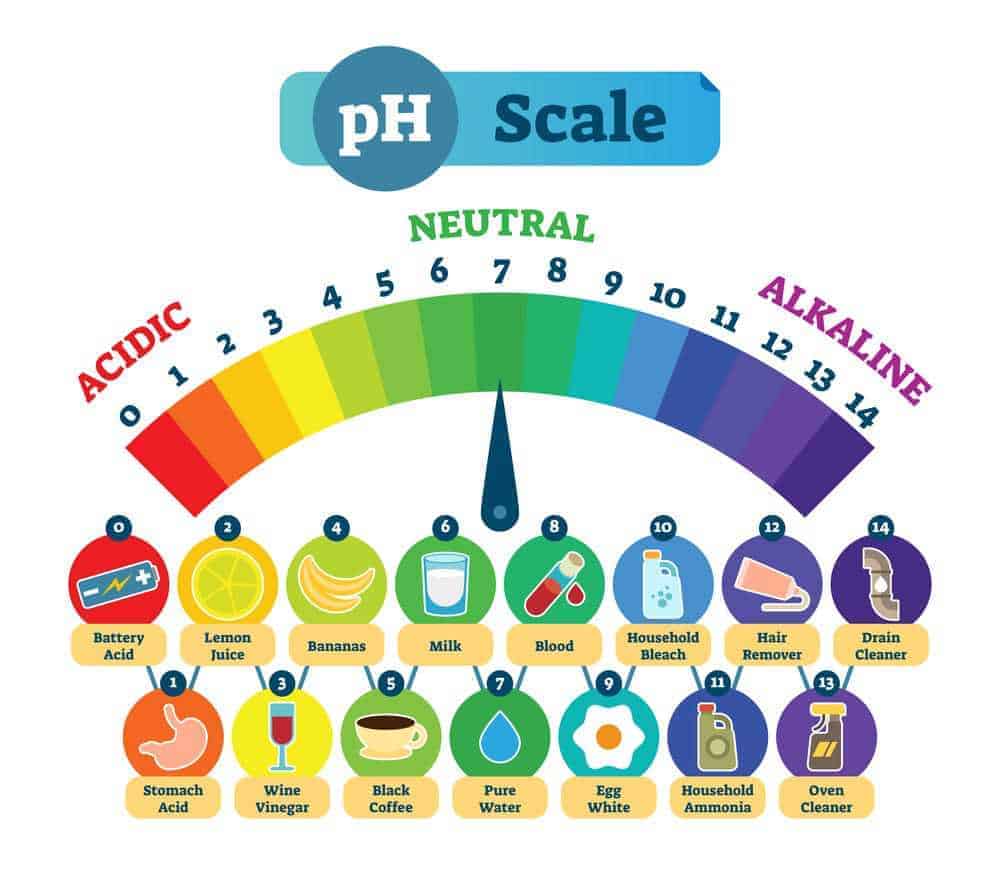
To sum up, what we discussed thus far, the pH scale is simply a measurement of the acidity and alkalinity of a substance – like a hair product. You will notice on the pH scale above that the range starts at 0 and goes up to 14. An acidic solution will have a pH of less than 7, while an alkaline solution will have a pH above 7.
The pH Scale Is a Logarithmic Scale
I don’t see this mentioned very often, but the pH scale is a logarithmic scale.
This means that a change in one whole number on the scale represents a tenfold change in potential hydrogen. For example, a pH of 6 is 10 times more alkaline than a pH of 5.
Each subsequent move across the scale represents an additional 10 times change in pH. For example, a change of two whole numbers (let’s say a pH of 5 to pH of 7) represents a change of 10 times 10. That’s a 100-fold change.
So, a pH of 7 (ex., distilled water) is 100 times more alkaline than a pH of 5 (ex., hair). This simply means that even small changes on the pH scale represent very large swings in the actual pH of a product.
Understanding logarithmic functions isn’t really common knowledge.
This causes many people to misunderstand the pH scale and misinterpret the meanings of the scale. That said, let’s discuss how the information we’ve discussed impacts your hair.

Healthy pH Balance and Hair Health
The concept of pH balancing is often confusing for even experienced ladies. Remember that pure water has a neutral pH of 7. This is an equal balance of hydrogen ions and hydroxide ions.
However, it’s not balanced when you compare it to your hair. The average pH of your hair is about 3.67 according to the International Journal of Trichology.
This means that a hair product with a pH of 7 is about 100 times more alkaline than a hair product with a pH of 5. The more alkaline a hair product is, the stronger and harsher it can be on the hair.
A hair product with a really high pH can leave your hair feeling dry, brittle, and porous. It’s ideal to use hair products that are closer to the pH of your hair with a Potential Hydrogen (pH) of 3.67.
How to Test pH of Hair Products
While Retesting Hair Products, Why Would I Receive Different pH Readings? Are the pH Strips Faulty?
It's possible that the pH strips are faulty. Although it's unlikely; we recommend purchasing pH strips from reputable sellers to mitigate the possibility of receiving faulty pH strips.
Most commercial pH strips work very well and remain effective for long periods but require proper storage - dark, dry storage areas are preferred.
How Accurate Are pH Strips?
The reality is that over-the-counter pH strips simply provide a general indication of pH level. There are highly accurate machines that are used in specific scientific studies which can measure a difference in pH between 6.988 and 6.989.
Over-the-counter pH strips usually measure pH in whole numbers, although there are some that attempt to be more precise. For example, if the pH level of a hair product is 6.5, it's likely to show either pH 6 or 7 on most commercial strips.
Again, there are some brands that will attempt to show the 6.5 reading. Basically, the pH strips are not highly specific, but they are still accurate enough when it comes to most do-it-yourself hair-related analyses.
Should I Purchase pH Strips That Provide Half Values? Are These Potential Hydrogen Strips Better?
Since these strips are only an estimate (often rounded) of the actual pH value, then I wouldn't search for any particular pH strips. Use whatever your favorite retailer carries or purchase pH strips online.
Frankly, if you're only using pH strips to test hair products, then whole number increments are sufficient. Since pH strips are usually pretty reliable, it's worth mentioning that user error could also cause faulty readings.
Only substances that contain water or substances that can dissolve in water have a pH reading. You'll also want to be careful with your hands while conducting pH tests.
Our hands happen to be a source of many chemicals in addition to soaps, lotions, and natural oils. If you are looking for a more accurate test result, avoid using your fingers to place the hair product or whatever you're testing on the pH strip.
The best way to achieve an accurate result is to use a glass rod or a clean spoon.
If you need to dissolve a hair product in water for your pH test, make sure that you use a glass that is clean. Plastic is known for leaching into a solution, while metal will also react with your solution, potentially causing inaccurate readings.

Testing the pH of Hair Conditioners
It's not possible to accurately test the pH of solids, and even the tiniest undissolved particles may affect the pH test. In other words, you can’t directly place a conditioner on a pH strip and then expect the result to be accurate.
A hair conditioner is not classified as a true liquid. For this reason, you need to dissolve the product in water and then conduct your test.
Keep in mind that solids will interact with the pH paper and can result in a reading that is false. However, when testing apple-cider vinegar or a water-glycerin mixture, these products should yield more accurate tests due to their water content.
Since Hair Isn't a Liquid, How Do You Test the pH of Your Hair?
To test the potential hydrogen (pH) of your hair, you need to soak the hair in deionized water for around 5 hours, followed by measuring the pH of your resulting solution. This test should be conducted at room temperature as pH is also sensitive to temperature.
The resulting liquid obtained through soaking your hair in deionized water will include protein, and trace elements, such as potassium, and calcium, and in some cases, traces from the environment like cigarette smoke.
In general, the pH will test for anything that is water soluble that comes out of the hair. These factors are the reason why the pH of hair and skin will vary so much (often between 3.5 and 5.5).
People use a variety of hair products, eat different foods, and are exposed to different regional elements depending on where they live.
Does pH Raise or Close Cuticles?
We’re familiar with two scientific studies that tested whether pH levels can raise hair cuticles or close cuticles. Both studies concluded that there is hardly any change to the hair structure between pH levels 4 to 9.
They concluded that hair structure remains constant despite changes in acid or base. The studies used hydrochloric acid, a very acidic substance, and the base hydroxide.
It was determined that the hair doesn’t absorb the hydrochloric acid or the base hydroxide between the pH levels of 4 and 10. Plus, between pH levels 4 and 10, cuticle separation was substantially unchanged.
Can High pH Levels Prevent the Cuticle from Opening?
It’s important to understand that cuticles aren’t like doors that open and close. It is a protein that can “lift,” but it doesn’t “open”. Research shows that the cuticle lifts due to a high pH of around 10.
What Happens When pH Is Below or Above the 3 to 9 Range?
When the pH is below 3, and above 9, the hair does seem to go through structural changes. This results in a change in density.
Researchers believe this is due to the acid and base changing the hair cuticle or protein. A very high pH level can actually cause the hair to dissolve.
Are You Able to Test the pH of a Substance Such as Coconut Oil?
No, and this is because coconut oil is not water-based, and it cannot dissolve in water. Therefore coconut oil cannot offer a potential hydrogen reading.
Does the pH of Hair Shift After a Person Uses Soap?
We weren’t able to find scientific evidence that the pH of hair changes while using soap, although it’s very likely.
According to the Journal of Cosmetics, washing your skin with water changes its pH levels; however, soap causes a more significant change in pH.
How Long Is the Recovery Time After Exposing Your Hair or Skin to Alkaline pH?
The recovery time will depend on a variety of factors. However, according to the British Journal of Dermatology, you should expect to wait anywhere from 45 minutes to 3 hours for your hair or skin to recover.
Do You Need to Worry About Irritation When Using Castile Soap or Baking Soda With an Alkaline pH?
Baking soda and castile soap can be irritating regardless of pH level. The irritation isn't coming from the soap itself. Soap helps water bond to oil on your body, but as a side-effect, it can remove your skin's protective oil layer leading to dryness and itchiness.
Even so, there is a strong relationship between pH and skin irritation. You shouldn't experience much irritation between pH 3 through 9, although there may still be a small, tolerable amount.
Once you drift outside of the pH range of 3 to 9, irritation levels can increase rapidly.
Related Articles:
Is Baby Shampoo pH Balanced?
It depends on the shampoo; however, there is quite a bit more to consider about the pH of baby shampoos. Shampoos, both for babies and adults, contain "surface active agents", commonly referred to as "surfactants".
One end of the surfactant molecule is repelled by water but attracted to oily substances, while the other is attracted to water. These molecules work by minimizing tension on the liquid's surface. This enables the shampoo to better spread and penetrate the hair.
In doing so, the surfactants dissolve "sebum", which is a thin layer of oil, from the hair and scalp. "No tear" shampoos for babies contain detergents that have long chain surfactants, such as nonionic polymers or sodium trideceth sulfate (STS).
These long-chain surfactants are far less harsh than normal detergents. However, only a small amount of these long-chain surfactants are used in "no tear" shampoos.
Additionally, formulas for babies which claim to be "tear-free" do not contain some of the more irritating surfactants, for example, sodium lauryl sulfate.
Sodium lauryl sulfate, which is made with palm kernel oil or coconut fat, is the chemical agent present in most adult shampoos which creates a lather. This cleans hair more thoroughly than the long-chain surfactants found in baby shampoo.
While they do still clean the hair, they are unable to remove oil as thoroughly. However, for babies, this is usually not an issue. They will often manage to get their hair incredibly dirty again immediately after getting out of the bath anyway.
You should consider making your own shampoo if you are in search of a pH balanced shampoo for babies that is almost entirely free of harsh chemicals. To do this, find a liquid castile soap (a soap that is made almost entirely from plant oils) and dilute it at a one-to-three ratio in water.
It is incredibly simple and cost-effective. But remember, even using the most chemical-free shampoo does not guarantee that bath time won't bring on at least a few tears.
Oftentimes, the pH balance in the water itself can cause tears due to the levels of hydrogen and hydroxyl ions present in the bath water.
Levels of pH are measured on a scale of 0 to 14. While 7 is neutral, a lower measurement of pH means that the water is less acidic. Any measurement that is greater than 7 means that the water is more acidic and contains a greater number of free hydrogen ions.
We generally recommend hair products with a pH of between 4 to 5, although for babies, our recommendation is different. Products with ranging from 6.5 to 7.6 will usually keep the bathwater "tear free".
Anything outside this range could be the cause of your baby's tears at bath time, not the shampoo itself.
The pH of hair plays a vital role in maintaining its structural integrity and protecting the hair from damage. With the hair shaft having a pH of around 3.67 and the scalp having a pH of 5.5, it is essential to choose hair products that align with these values.
Using pH-balanced products can help prevent cuticle damage and other hair issues. Keep in mind that the pH scale is logarithmic, meaning even small changes can have significant effects on hair health. To ensure you are using the best products for your hair, consider testing their pH levels and selecting those that closely match your hair's natural pH.




